Engineer Multi-Dimensional Cell Membrane-Coated Nanomaterials to Combat Bacterial Infection
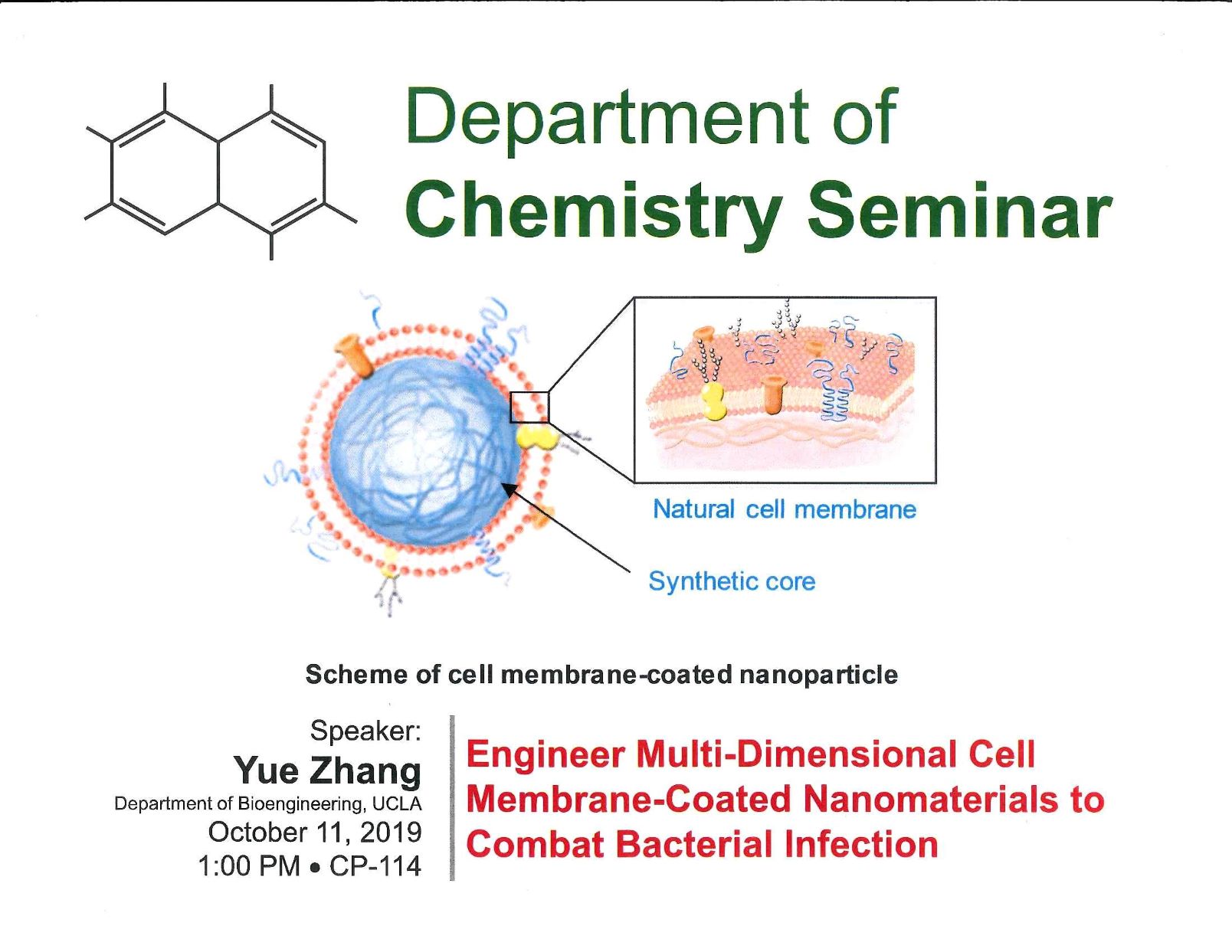
Abstract: Designing a biomimetic nanoparticle is a challenging topic. Despite the advances in surface chemistry and material science, it’s still impossible to fully replicate the complex interface that presents in nature. Besides, the bottom-up method fails to prevent the exposure of foreign material when administrated in vivo. To solve this challenge, we directly fuse the natural cell membrane onto the synthetic cores. This top-down method quickly generates natural stealth for the synthetic material and preserves the biofunction from the resource cell. Due to its unique properties, cell membrane coating technology displays great potential in the field of drug delivery, detoxification, vaccination and biosensing. In this talk, we will be discussing the applications of cell-membrane coated nanoparticles (also known as “nanosponge”) in treating bacterial infection. Instead of direct inhibiting the bacteria growth, nanosponges neutralize the virulence factors that bacteria secrete to subvert immune surveillance, which is known to place less evolutionary pressure in inducing antibiotic resistance. In the first thrust, antivirulence efficacy is carried out solely by biomimetic nanosponges. Two types of cell membrane from different origins are explored in this direction. In the second thrust, nanosponges are mixed with the supporting material to form a stable composite, which in turn boosts the antivirulence efficacy of the nanosponge. Together, cell membrane coating technology offers us a great tool to solve the clinical challenges by mimicking the biological events happening at the natural biointerface.
Thinking Cells as Macromolecules: A Chemist's Pondering Upon Cell Biology

Abstract: Conventionally physical chemistry is a field that mainly investigates physicochemical phenomena at atomic and molecular levels. Noticing the analogy between molecular (especially macromolecular) dynamics and cellular dynamics, in the past few years my lab has focused on introducing and generalizin
g the techniques and concepts of physical chemistry into cell biology studies. In this talk I will first discuss a long-standing Nobel-Prize winning puzzle on olfaction. Each olfactory sensory neuron stochastically expresses one and only one type of olfactory receptors, but the molecular mechanism remained unanswered for decades. I will show how simple physics taught in introductory physical chemistry textbook explains this seemingly complex problem, and briefly mention our ongoing efforts of investigating chromosome dynamics with a CRISPR-dCas9-based live cell imaging platform.
In the second part of my talk, I will discuss our efforts on developing an emerging new field of single cell studies of the dynamics of cell phenotypic transition (CPT) processes, in parallel to single molecule studies in chemistry. Mammalian cells assume different phenotypes that can have drastically different morphology and gene expression patterns, and can change between distinct phenotypes when subject to specific stimulation and microenvironment. Some examples include stem cell differentiation, induced reprogramming (e.g., iPSC) and others. In many aspects a CPT process is analogous to a chemical reaction. Using the epithelial-to-mesenchymal transition as a model system, I will present an integrated experimental-computational platform, and introduce concepts from chemical rate theories such as transition state, transition path, and reactive/nonreactive trajectories to quantitatively study the dynamcis of CPT processes.
Research: https://www.csb.pitt.edu/Faculty/xing/
Book Release Celebration for Jayhawker: On History, Home, and Basketball

$3 Million NSF Grant to Support the Next Generation of Kentucky Food, Energy and Water Systems Innovators
By Dave Melanson
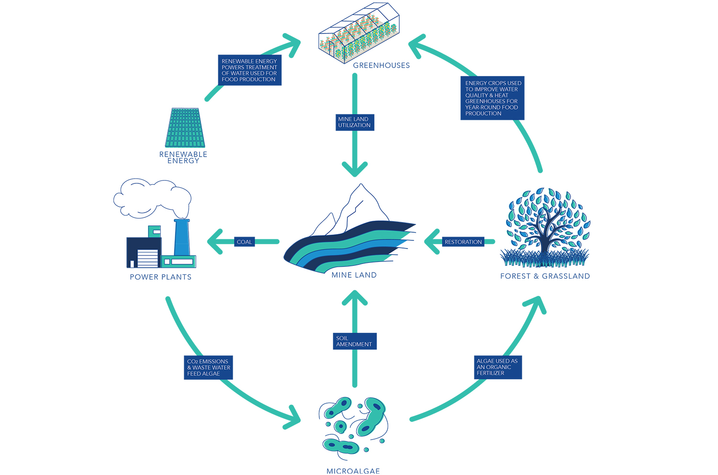
This project will combine graduate student training with cutting-edge research in mine land remediation, water treatment, crop production and power generation and will help address the need for innovators in food, energy and water systems.
College of Arts and Sciences to Induct 6 New Members at 20th Hall of Fame Ceremony
By Jenny Wells-Hosley
The University of Kentucky College of Arts and Sciences will induct six new members into its Hall of Fame this week.
This year marks the Hall of Fame’s 20th anniversary and the induction ceremony will take place at 6 p.m. Friday, Oct. 4, in the Gatton Student Center's Worsham Cinema.
This year's honorees include:
Alumni Inductees:
UK Team Selected by Robert Wood Johnson Foundation to Address Children's Health Challenges in Casey County
By Jenny Wells-Hosley

The Robert Wood Johnson Foundation has selected five UK professors to serve in its Clinical Scholars program. From left: Craig Miller, Angela Grubbs, Julie Plasencia, Audrey Darville and Charles Carlson. Photo by Renee Fox.
4th Annual BioBonanza to Showcase UK Research
By Jillian Gibney

UK's Department of Biology's annual open house festival will take place 11 a.m. to 2 p.m. Saturday, Oct. 5, at the Don and Cathy Jacobs Science Building.
Join the University of Kentucky Department of Biology for its fourth annual BioBonanza, a one-day open house festival, from 11 a.m. to 2 p.m. Saturday, Oct. 5, at the Don and Cathy Jacobs Science Building (600 Rose St., Lexington).
UK Freshman Math Major is Speedcubing World Record Holder
By Jenny Wells-Hosley
University of Kentucky freshman Lucas Etter still remembers the first time he solved a Rubik's cube — it was exactly 10 years ago to this day.
"I first found a Rubik's cube in my grandparents' basement when I was 8, and my first time completing it was Sept. 27, 2009. So, I have been solving the cube for over half my life."

