"Metabolic Regulation of the Male Germline Stem Cell Niche"
 Dr. Rafael Demarco | Demarco Lab
Dr. Rafael Demarco | Demarco Lab
Bio:
I am a new Assistant Professor in the Department of Biology at the University of Louisville whose ultimate goal is to understand how changes in metabolism impact stem cell behavior during homeostasis, aging and stress conditions. I was trained as a geneticist during my Ph.D. with Dr. Erik Lundquist at the University of Kansas, where I learned to ask questions and interpret genetic data using model organisms. To pursue my objective of studying stem cells and their niches, I obtained my postdoctoral training and later position as a Research Specialist in the laboratory of Dr. Leanne Jones (first at the Salk Institute and then at the University of California, Los Angeles and San Francisco), a leading expert in the fields of stem cells and current director of the Bakar Aging Research Institute at UCSF. During my time working with Dr. Jones, I developed my own research interests focusing on how different aspects of metabolism impact the stem cell niche present in the Drosophila testis. Unexpectedly, I found that both stem cell populations present in the testis niche employ mechanisms to maintain proper lipid homeostasis in order to prevent stem cell loss. Disruptions in either mitochondrial fusion (in germline stem cells1) or autophagy (in cyst stem cells2) led to deficient lipid catabolism and ectopic accumulation of lipids in the stem cell niche, which promoted stem cell loss through differentiation. Hence, a model has emerged revealing a novel metabolic facet in the regulation of stem cell fate, which appears conserved across stem cell systems3. In my recently established laboratory, I am engaged in pursuing the mechanism(s) through which ectopic lipid accumulation can impact stem cell fate within the niche, which could shed light into the development of new strategies targeting stem cell-based regenerative therapies.
Abstract:
The capacity of stem cells to self-renew or differentiate has been attributed to distinct metabolic states. A genetic screen targeting regulators of mitochondrial dynamics revealed that mitochondrial fusion is required for male germline stem cell (GSC) maintenance in Drosophila melanogaster. Depletion of Mitofusin (dMfn) or Optic atrophy 1 (Opa1) led to dysfunctional mitochondria, activation of Target of Rapamycin (TOR), and a dramatic accumulation of lipid droplets (LDs). Pharmacologic or genetic enhancement of lipid utilization by the mitochondria decreased LD accumulation, attenuated TOR activation and rescued GSC loss caused by inhibition of mitochondrial fusion. However, the mechanism(s) leading to GSC loss were unclear. TOR activation has been demonstrated to suppress JAK-STAT signaling by stabilizing the JAK-STAT inhibitor SOCS36E. As JAK-STAT signaling is critical for regulating stem cell self-renewal in the testis, we wanted to test the hypothesis that the increase in TOR activity in early germ cells would lead to SOCS36E stabilization, which in turn, could contribute to stem cell loss. Indeed, we found that SOCS36E levels were higher in early germ cells upon depletion of dMfn or Opa1. Subsequently, we show that activation of the JAK-STAT pathway, but not BMP signaling, is sufficient to rescue loss of GSCs as a result of the block in mitochondrial fusion. In addition, preliminary genetic and proximity-labeling data suggest that LD accumulation acts in parallel to TOR/SOCS36E to promote GSC loss. Our findings highlight a critical role for mitochondrial metabolism and lipid homeostasis in GSC maintenance, providing a framework for investigating the impact of metabolic diseases on stem cell function and tissue homeostasis.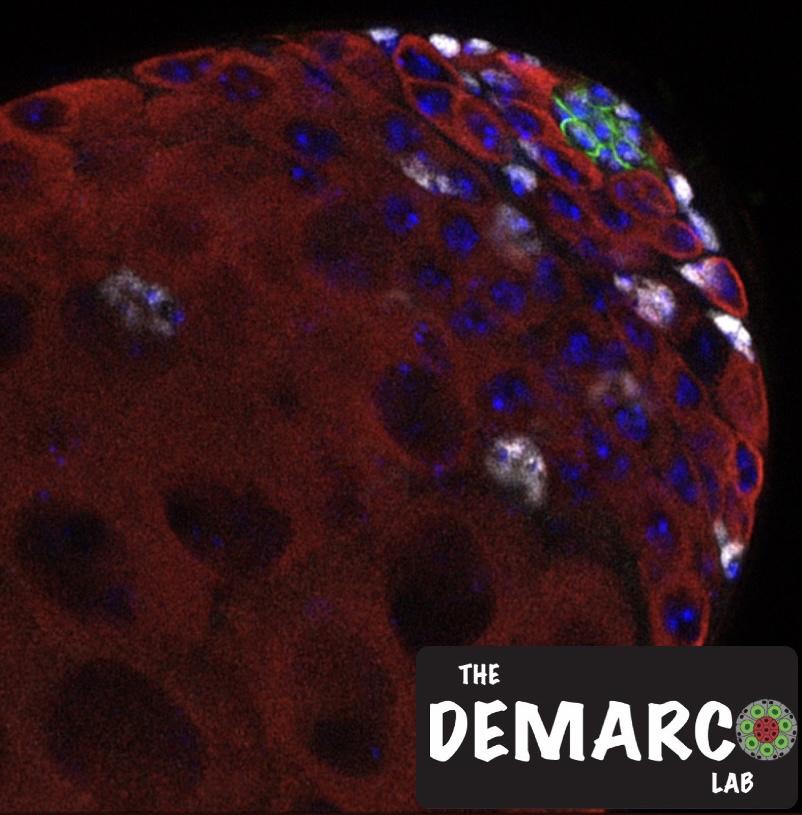


 Hugo Reyes-Centeno
Hugo Reyes-Centeno