 Sachin Handa
Sachin Handa
Prof. Sachin Handa is currently a tenured associate professor in the chemistry department at the University of Louisville. In less than four years, he completed his Ph.D. in 2013 and then worked as a postdoc fellow with Prof. Bruce Lipshutz from 2013-2016. He started his independent career in 2016. His research interests are green chemistry, energy, nanocatalysis, and photochemistry. Recently, he has received the NSF CAREER award, Ralph E. Powe Junior Faculty Enhancement Award in Physical Sciences by Oak Ridge Associated Universities, and Peter J. Dunn Award for Green Chemistry and Engineering Impact in the Pharmaceutical Industry. Besides fundamental research, his research significantly focuses on synthetic problems associated with the pharmaceutical industry. Currently, his research is funded by NSF, Novartis Institutes for BioMedical Research, Novartis Pharmaceuticals Switzerland, AbbVie, Takeda, and Biohaven Pharmaceuticals. He also serves on editorial boards of various journals, such as ACS Sustainable Chemistry and Engineering, Green Chemistry Letters & Reviews, and Molecules.
Abstract
Water appears to be the only solvent for life to occur on this planet; nonaqueous solvents may support life elsewhere in the universe.
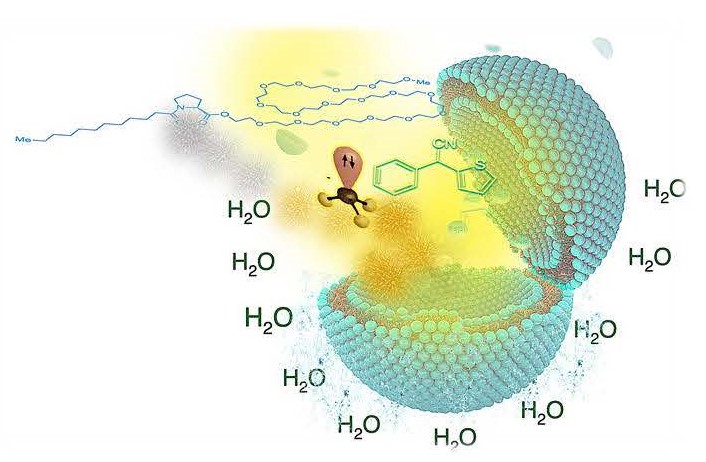
Water is a safe, stable, inexpensive, and naturally abundant solvent. However, it is predominantly used for reaction work-ups in organic synthesis rather than as an alternative reaction medium. Nonetheless, it has many exciting features leading to more effective and environmentally cleaner chemistry, such as enhancing catalysis and controlling reaction selectivity via metal-micelle cooperativity or the shielding effect of the micelle.Therefore, in the big picture, the effective use of water in syntheses enables powerful catalysis that can avoid expensive ligands and the use of toxic organic solvents, boost the worker and environmental safety, and add tremendous economic value. Contributions from various research groups, and ours, have set the foundation for adoption of chemistry in water for academic and, especially, for industrial applications. After all, with nothing to lose and everything to gain, chemistry in water can be the future.
In this talk, therefore, the focus will be on:
• Why is there a huge need for effective green chemistry research?
• How can chemistry in water provide practical solutions to ongoing and future problems in synthetic process chemistry?
• A fundamental understanding of how nanomicellear catalysis works
• The design of sustainable nanocatalysts via synergies between water, metals, and micelles
• Reaction selectivity and enhanced stability of water-sensitive intermediate’s arising from metal-micelle cooperativity for sustainable carboxylation, amination, amidations, and other reactions.
Faculty Host: Dr. Robert Grossman


