By Richard LeComte
 LEXINGTON, Ky. – Lift up a rock by a lake, and you may find planarians: complex, light-hating flatworms that prefer damp, dark hideouts. But what has Elizabeth Duncan so interested in them is their remarkable ability to reconstruct themselves when severed or injured.
LEXINGTON, Ky. – Lift up a rock by a lake, and you may find planarians: complex, light-hating flatworms that prefer damp, dark hideouts. But what has Elizabeth Duncan so interested in them is their remarkable ability to reconstruct themselves when severed or injured.
“If we cut one of these worms into three pieces, each piece will create an entirely new worm,” said Duncan, assistant professor of biology in the University of Kentucky’s College of Arts & Sciences. “They regenerate a fairly complex body plan.”
That ability may lead Duncan and other researchers to fresh insights into biological regeneration. As a result, she recently earned a five-year, $250,000-a-year National Institute of General Medical Sciences Maximizing Investigators’ Research Award. The award will help her explore how specific enzyme activity affects stem cells’ ability to reproduce a viable organism and how it relates to human development.
Planarians’ ability to regenerate means they can live on essentially forever unless they hit an adverse environment.
“If you keep them healthy, if their water is not polluted with poison or contaminated with bacteria, they can live forever,” Duncan said. “We call them negligibly senescent, which means they don’t really age. Which is crazy, right?”
So how do they regenerate themselves, and what happens when scientists interfere with the process? Duncan and her research team can go into the planarian and “turn off” certain enzymes by removing the mRNA that encodes them. That change can alter essential processes when the worm regenerates: For example, the reconstructed worm could lose a great deal of its cilia, which it needs to move in and sense its surroundings.
“When I started my postdoc, I studied transcription: essentially, how the packaging of DNA affects the transcription of genes,” she said. “I came into the planarian field, and I found that there was just not that much known about how those enzyme systems work in planarians.”
Duncan and her colleagues study enzymes responsible for the methylation (a transfer of a methyl group in DNA) of Histone H3 at lysine 4, or H3K4me3. In other animals, including humans, this modification is highly abundant on active genes and often undetectable in inactive genes. One would predict that removal of the enzymes that add this “activation” mark would render those genes ineffective, but in reality it’s not that simple.
“What is mysterious is that in vivo, if you get rid of it (H3K4me3), it doesn’t always turn the gene off,” she said. “In most organisms, you’ll see this high correlation between H3K4me3 and gene expression. But if you remove it, a lot of the time the gene turns off, but sometimes it doesn’t. We realized we had to understand what’s going on in an organism, and we think that it has to do with when a cell turns into the next cell type. If you get rid of H3K4me3, you may not lose gene expression, but if you ask that cell to turn into an entirely different cell type, then it’s a problem.”
In planarians, you can deplete the mRNA of specific genes and induce the organism to regenerate. But they might not do so correctly. For example, they can lose their ability to glide smoothly across a surface, such as the bottom of a petri dish.
When you individually deplete the enzymes that add H3K4me3 to specific genes, “the planarians have several different phenotypes,” she said. “One basically makes the worm go from immortal to mortal. You lose the stem cells over time, and they don’t regenerate.”
But when members of Duncan’s lab deplete the second H3K4me3 enzyme, “the planarians really struggle to get away from the light, even though they also want to,” she said. “And it turns out it’s because the cilia on the epithelium is gone.”
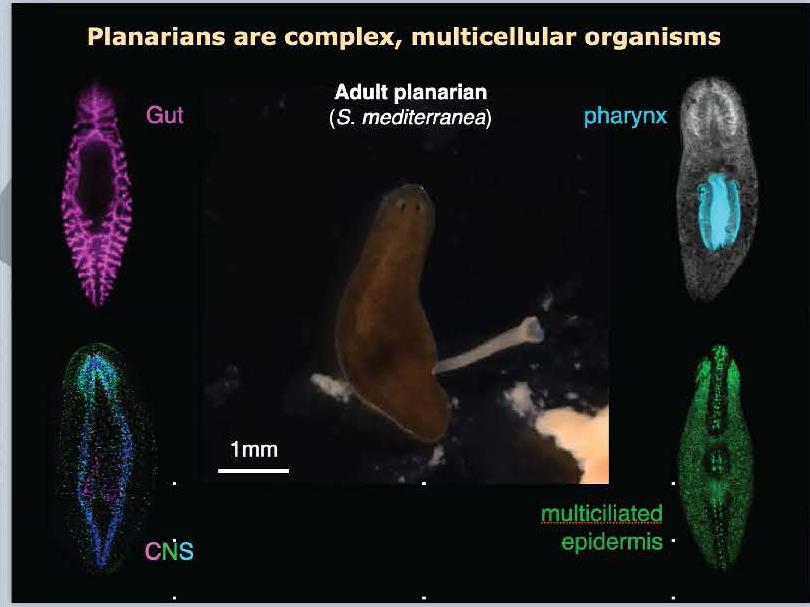 Duncan’s current research aims to understand how planarian stem cells use this enzyme to prepare them to turn into ciliated cells. Removing the enzyme affects the stem cells’ decision to become ciliated cells once they reach the epidermis of the organism. Duncan points to hints in research publications studying other animals, including human patients, that suggest this enzyme process is also an important factor in how stem cells behave during early embryonic development, particularly that of the human heart.
Duncan’s current research aims to understand how planarian stem cells use this enzyme to prepare them to turn into ciliated cells. Removing the enzyme affects the stem cells’ decision to become ciliated cells once they reach the epidermis of the organism. Duncan points to hints in research publications studying other animals, including human patients, that suggest this enzyme process is also an important factor in how stem cells behave during early embryonic development, particularly that of the human heart.
Using the technique with which they can turn off the genes, Duncan hopes her research may lead to identifying genes that are involved in various human processes and disorders. Because they are turning the genes off in adult planarian worms, they do not need to worry about killing the animal before it is even born. Planarians’ ability to regenerate allows the Duncan lab to induce stem cell differentiation and organ development in the absence of individual genes, even when a given gene is essential to the earliest stages of these processes.
“We’re interested in trying to use our model to screen different genes,” she said. “Over the summer undergrads in my lab cloned the genes, then screened them to see if a gene is important or not.”
In addition, the turnaround time for these experiments is dramatically shorter than comparable experiments in vertebrate models. The process of knocking down a gene usually takes approximately two weeks, followed by the two weeks it takes a tissue fragment to regenerate a new worm. The Duncan lab routinely screens dozens of genes per month using this method.
“We can certainly screen through a lot more genes this way,” she said.
Although the COVID pandemic was disruptive to this young lab, Duncan tried to come up with remote projects for her students that would be useful once they got back to the bench. For example, several undergraduate research students created a database of human diseases associated with genes targeted by planarian H3K4me3 enzymes.
Now she and her students can screen genes based on the human disease with which they are associated, “we can look at our list of worm genes and then compare it to a human database and ask, ‘What happens in a human patient if this gene is mutated?’ Some of the effects are connected to heart defects, but we also see connections with cancer.”
Duncan is excited to see what unexpected discoveries they will find with this approach.
“Many medically relevant discoveries started with key observations made in invertebrate organisms: restriction enzymes, telomerase, CRISPR, genes that regulate cell proliferation,” she said. “Planarians have so many incredible abilities, they must be able to teach us something.”
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM142679. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
