Date:
-
Location:
CP 114
Speaker(s) / Presenter(s):
Dr. Bruce Palfey
----------
Dr. Bruce Palfey from the University of Michigan will be presenting a seminar titled: A New Redox State in Flavin Enzymology.
Faculty Host: Anne-Frances Miller
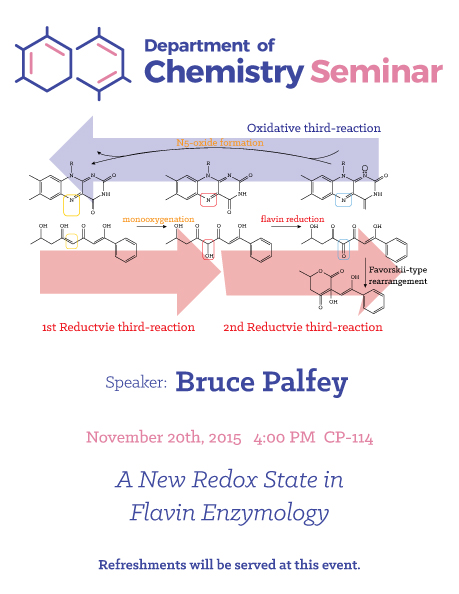
Abstract
Look in a good biochemistry textbook. It will mention three oxidation states for flavins: fully oxidized, one-electron reduced, and two-electron reduced. These redox forms have been studied since before you were born, and account for a huge amount of the chemistry of flavoenzymes; highly unstable covalent adducts to flavins account for the rest. In particular, a plethora of flavin-dependent monooxygenases have been identified. Back in the age of disco and into the Reagan administration, there was a hot debate over the identity of the flavin intermediate that oxygenates organic substrates. The flavin-N5- oxide, known from chemical synthesis, was proposed as the intermediate, but resoundingly rejected - only the flavin-C4a-hydroperoxide was clearly consistent will all experimental evidence. Thus the flavin-N5-oxide was banished from enzymology - until now. The enzyme EncM is part of the enterocin biosynthetic pathway. It uses O2 to
oxygenate a complex polyketide substrate. Years of searching for a flavin-C4a- hydroperoxide oxygenating intermediate were fruitless. Instead, we were shocked - yes, shocked! - to unequivocally identify the flavin-N5-oxide as the species that transferred oxygen to the substrate [1]. This identification rests on mass spectroscopy, isotopic labeling, absorbance spectroscopy, and good old fashioned chemistry [1, 2]. Its discovery leads to a catalytic cycle that is very unusual for a flavoenzyme. With a cool new enzyme intermediate come many questions. What is the mechanism of its formation? What is the mechanism it uses for oxygen-transfer? What kinds of substrates can it oxygenate? Are there any other enzymes that use the flavin-N5-oxide? Etc.